ABOUT US
DAPI’s Mission
DAPI - The German Institute for Drug Use Evaluation (Deutsches Arzneiprüfungsinstitut e. V. (DAPI)) is an association registered under number VR 38247 B in the Vereinsregister (Register of Associations) of the Charlottenburg Amtsgericht (District Court) in Berlin and is recognized as a non-profit association by the Finanzamt Körperschaften I (Tax Office for Corporations), Berlin.
DAPI concerns itself with the pharmacoeconomic and pharmacoepidemiological assessment and evaluation of drugs as well as general questions of drug supply. To this end, analyses of pharmacy claims data from the statutory health insurance (GKV) are carried out and are reported on. According to the statutes of the association, the aim of the association is to promote science and research as well as the promotion of public health and public health care. This is realised through the establishment, maintenance and management of an institute to assess and evaluate drugs.
DAPI handles all questions related to the assessment and evaluation of drugs and health products in a scientific manner, supports health authorities and institutions in the health sector in the field of drug supply and, in particular, carries out assessments and prepares reports with the aim of improving safety and the securing, and further developing of, quality in medical care.
The DAPI Data Warehouse
Around 90% of the German population have statutory health insurance (SHI).
The reports, analyses and studies of DAPI are based on its own database (data warehouse) with prescription data for drugs and other medical products that were dispensed in community pharmacies in Germany at the expense of the statutory health insurance. Since 2000, DAPI has received data from currently six large data processing centres in all regions of the Federal Republic of Germany. Efforts are being made to integrate further data centres.
The DAPI database contains data from a representative sample of more than 95% of the statutory health insurance drug billings from community pharmacies in Germany since July 2019. Until June 2019, the representative sample was over 80%. The data are then extrapolated with regional factors to achieve 100% of all drug dispensings for statutorily insured persons. Until June 2019, the extrapolation method was based on the number of community pharmacies in the 17 regions of the State Chambers of Pharmacists. The regional factors for this period were calculated annually on a fixed date from the proportion of pharmacies recorded in the DAPI database with regard to all pharmacies in the respective region. From July 2019 onwards, the extrapolation is based on the regional sales of drugs billed to the SHI. The quarterly reports of the GKV-Arzneimittel-Schnellinformation (GAmSi) for the respective region serve as a reference for 100%. The regional factors are updated regularly and are calculated from the share of the dispensed packs recorded in the DAPI database for the respective region in relation to the pack numbers given in the GAmSi reports.
DAPI thus has a database that is unique in terms of the completeness of statutorily insured persons over such a time period. The database contains around 13 billion prescriptions from statutory health insurance claims and is growing by over 700 million entries annually. The data are anonymized, i.e. they contain no reference to individual insured persons, doctors or pharmacies. DAPI receives the data on a monthly basis as a copy of the accounting data that has been anonymized (with regard to personal data) by the data centres. Drug prescription data are loaded into the DAPI data warehouse, along with drug information updated every 14 days from ABDATA Pharma-Daten-Service, Eschborn. The data are then available for analysis (see Figure 1).
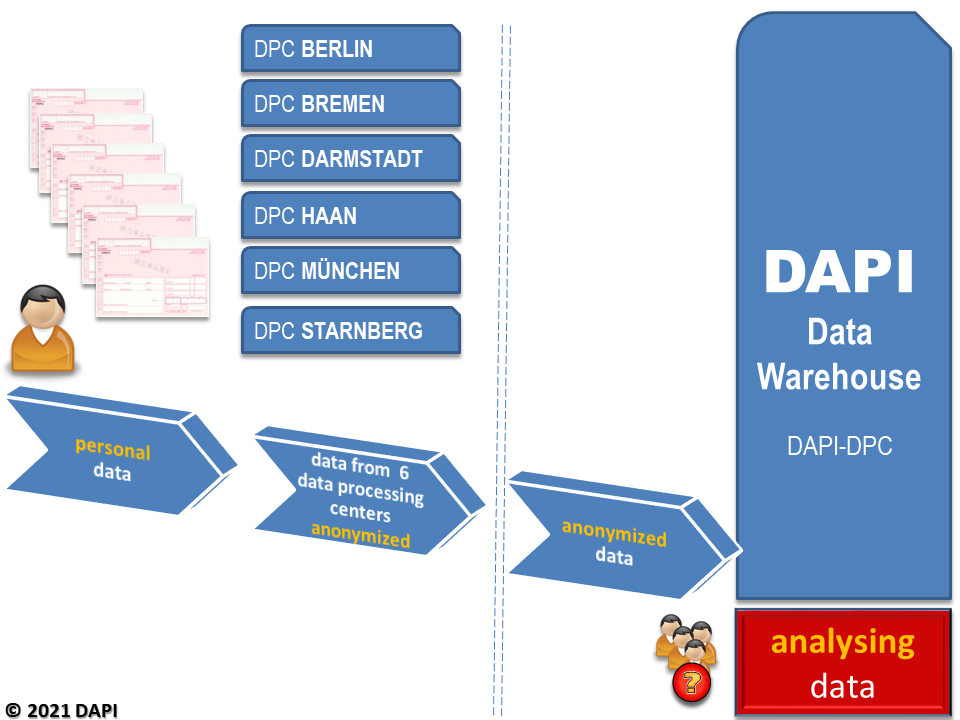
Figure 1: Processing of statutory health insurance drug prescription data from public pharmacies via data processing centres is loaded into the DAPI database (data warehouse)
What Data does DAPI have available?
The information marked in Figure 2 from the statutory health insurance prescription is loaded into the DAPI database.
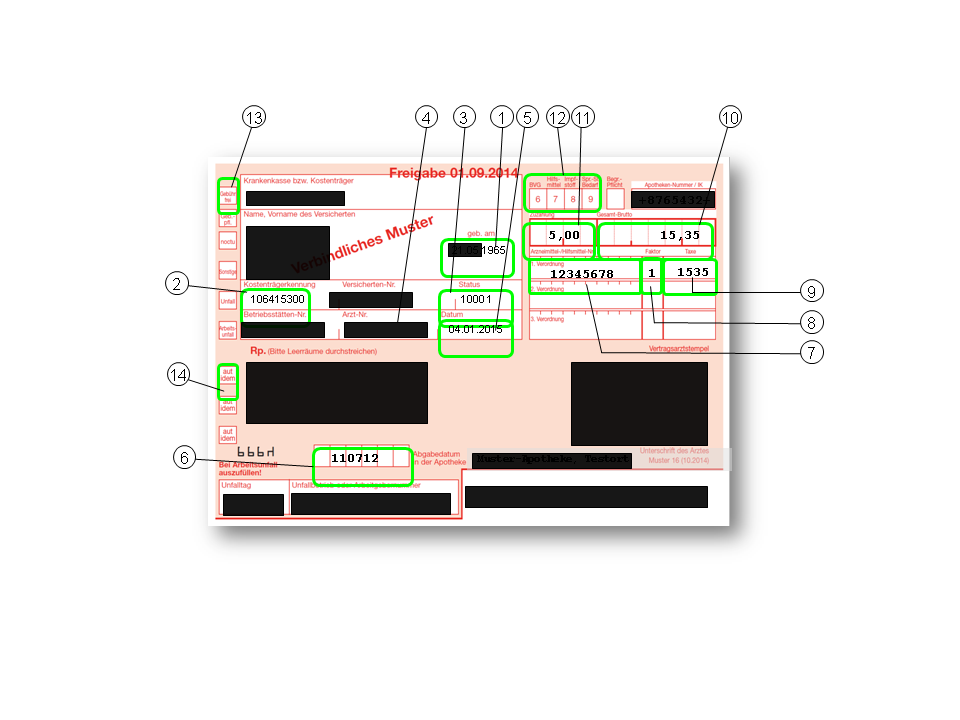
Figure 2: SHI prescription form (so-called “Muster 16” (Sample 16)). Green borders show information that is available in the DAPI database. Information that is not available is blacked out.
The most important billing information includes:
1) Year of birth of the patient (clustered into annual groups)
2) Health insurance provider (statutory only)
3) Status of the insured person (e.g. member, family insured person, pensioner)
4) Health insurance provider region of the prescribing doctor (17 KV regions (Kassenärztliche Vereinigungen (Regional Associations of Statutory Health Insurance Accredited Physicians)); most of these represent the 16 federal states of Germany); extracted from the field (4) )
5) Date of issue
6) Date of dispensing in the pharmacy
7) "Pharmazentralnummer" (PZN) of the prescription according to § 300 SGB (Sozialgesetzbuch (Code of Social Law)) V. The PZN is the standard federal identification key for drugs, domiciliary equipment and other pharmacy products. It is an eight-digit number that uniquely identifies the drug according to its name, dosage form, strength of active ingredient and pack size. ‘Domiciliary Equipment Position Number’ of the prescription according to § 302 SGB (Sozialgesetzbuch (Code of Social Law)) Hilfsmittelpositionsnummer (Domiciliary Equipment Position Number): Standard federal national identification key for adomiciliary equipment according to product group, place of use, subgroup, type of use and product
8) Number of packs dispensed
9) Charge (declared price) of the product dispensed
10) Total price for all products on the prescription
11) Co-payment by the patient
Information about whether e.g. ...
12)
... the prescription is a vaccine or was prescribed for consultation overhead supplies
... the prescription was due to an workplace accident
... the prescription was prescribed during night and emergency services
13) … the insured person is exempt from the co-payment
14) … the prescription line has been marked "Aut idem" (therapeutic substitution)
The prescription data in the DAPI database (data warehouse) are linked to the ABDA database, which contains a complete directory of all German medicinal products as well as domiciliary equipment and other products available in pharmacies. The connection between the two databases is made with the PZN. The current ABDA database is loaded into the DAPI database every 14 days. This makes it possible to take a historicised look at changes (e.g. price) in medicinal products over time. A link between the prescription data and information about the product valid at the time of the prescription is thus guaranteed.
The most important information from the ABDA database includes:
- Trade name of the drug
- Dosage form (e.g. tablet, capsule, ointment,...)
- Pack size (e.g. number of separate units, weight or volume of the pack) and unit (e.g. piece, mg, ml)
- Ingredients of the product, including quantities of the active ingredients per divided unit (strength)
- Classification of active ingredients according to ATC code (anatomical-therapeutic-chemical classification system for drugs, published by the World Health Organization (WHO))
- Defined daily dose (DDD)
- ABDATA indication areas (mixed classification according to chemical, pharmacological and therapeutic aspects)
- Drug status: drug, narcotic, biotech drug, prescription drug, imported drug, discount drug, etc.
- Price information: Pharmacy purchase price, VAT rate, pharmacy retail price, discounts and rebates, manufacturer's rebate, reimbursement amounts, fixed amounts.
- Information about the provider
It should be noted that the DAPI database only includes prescriptions covered by the statutory insurance providers that were dispensed in community pharmacies. Information on drugs dispensed as part of self-medication (OTC), drugs/products prescribed on private prescriptions (in Germany, approx. 10% of the population is privately insured) and drugs for patients during a hospital stay are not included. Furthermore, there is no information on diagnoses or, for example, laboratory data in the DAPI database.
Which questions does DAPI work on and for whom?
DAPI provides its member organizations with regularly updated basic and routine evaluations from its database. The basic evaluations enable the member organizations to compile market overviews according to a wide variety of criteria, for example, according to drug groups (ATC code) or individual products (PZN). Routine evaluations deal with special topics such as the dispensing of vaccines or magistral preparations and provide partial results according to (insurer) regions and the types of health insurance funds (AOK, BKK, BEK, DAK, ...). The ‘Number of the Month’ section makes analysis available on a wide variety of topics that are of general interest.
For particular questions, DAPI can perform evaluations on request. Important analytical results are published not only on our website but also in national and international journals to make them available to a wider specialist audience.
The questions addressed to DAPI are manifold; our evaluations are always scientifically sound and independent. The spectrum of potential evaluation recipients extends far beyond DAPI members: health ministries and authorities, health politicians, members of parliament, health insurance companies, health care institutions, scientific institutes and individuals working in health care professions.
Inquirers undertake to pass on analysis results to third parties only with the express written permission of DAPI. Inquirers may publish analytical results only after consultation and with written permission of the DAPI. The results of analyses may only be used if the source is mentioned ("© Deutsches Arzneiprüfungsinstitut e. V. (DAPI), Berlin"). The reproduction and use of the results of the data analysis must be done in such a way that the results of the data analysis are factually correct.

In October 2011, das Deutsche Arzneiprüfungsinstitut (DAPI) e. V. was included in the database of the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) as a research centre for pharmacoepidemiology and pharmacovigilance. Link to DAPI-Entry
ENCePP is a project of the European Medicines Agency (EMA), London, with the aim of improving research into post-authorization drug use by pooling the expertise available in Europe and facilitating the multicentre and independent studies on the benefits and risks of medicines after they have been launched. Key activities of ENCePP include the maintenance of a publicly accessible database containing information on European research centres, networks and databases, as well as the establishment and maintenance of an electronic study registry. Furthermore, a Code of Conduct was established, which defines essential rules and principles as a standard for pharmacoepidemiological research activities. This includes the principles of transparency and scientific independence as well as regulations for the publication of study results. In addition, guidelines on methodological standards in pharmacoepidemiology were developed, which represents the current scientific state in the field.
DAPI is committed to adhering to the methodological standards of the "ENCePP Guide on Methodological Standards" and to promoting scientific independence and transparency. DAPI studies are registered in the ENCePP electronic registry.
Protection of data privacy
Data protection and preservation of anonymity, especially of the insured, are top priorities for DAPI. The legal basis for the statistics and surveys is Section 300 (2) SGB (Sozialgesetzbuch (Code of Social Law)) V, which permits the processing and use of anonymized data from statutory health insurance drug billing.
In the case of inquiries to DAPI, only processed analysis and results derived from the underlying data are passed on. The forwarding of original data records from DAPI's own database is not allowed. The query strategy, the results of the data analyses, and explanations necessary to explain the analyses and/or conclusions derived from them are the intellectual property of DAPI.
DAPI is in contact with the responsible data protection authorities or data protection officers and coordinates its analysis options with them.